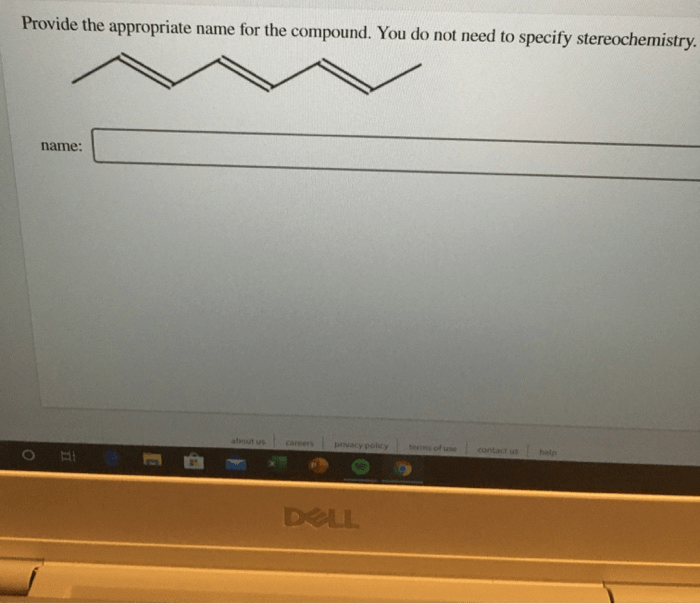
Give the systematic name of each covalent compound. spelling counts – The systematic naming of covalent compounds, as Artikeld by the International Union of Pure and Applied Chemistry (IUPAC), is a fundamental aspect of chemistry that ensures clear and consistent communication within the scientific community. This guide delves into the principles and importance of accurate spelling in systematic naming, highlighting common errors and providing strategies to enhance precision.
By adhering to IUPAC guidelines and paying meticulous attention to spelling, chemists can effectively convey the structure and identity of covalent compounds, enabling accurate interpretation and facilitating collaboration.
Systematic Naming of Covalent Compounds: Give The Systematic Name Of Each Covalent Compound. Spelling Counts
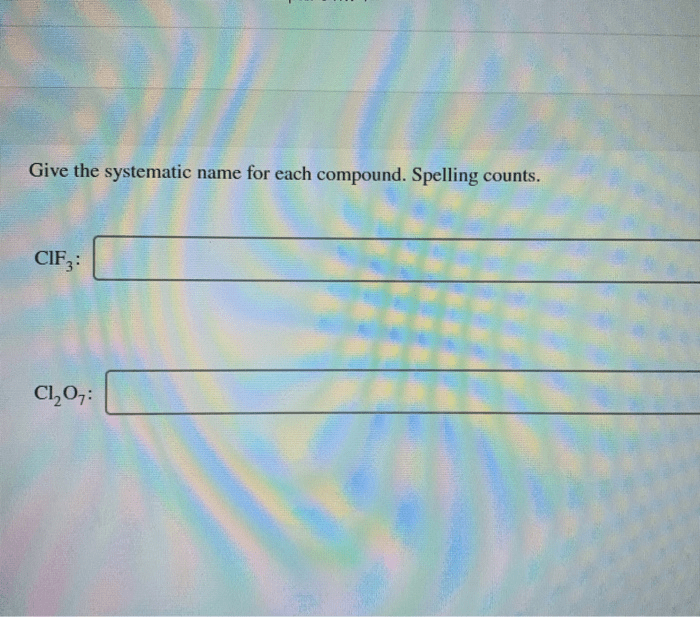
The International Union of Pure and Applied Chemistry (IUPAC) has established a systematic nomenclature system for naming covalent compounds. This system provides a consistent and unambiguous way to identify and describe these compounds.
To name a covalent compound, the following steps are generally followed:
- Identify the central atom.
- Identify the ligands attached to the central atom.
- Use the prefixes “mono-“, “di-“, “tri-“, “tetra-“, etc., to indicate the number of each type of ligand.
- Use the suffix “-ide” for the ligands.
- Combine the prefixes and ligands to form the name of the compound.
For example, the systematic name for the covalent compound CH4 is methane. The central atom is carbon, and the four ligands are hydrogen atoms. The prefix “mono-” is not used for the hydrogen atoms because it is understood that there is only one of each type of ligand.
The systematic name for the covalent compound C2H6 is ethane. The central atom is carbon, and the six ligands are hydrogen atoms. The prefix “di-” is used for the hydrogen atoms because there are two of them.
The systematic name for the covalent compound NH3 is ammonia. The central atom is nitrogen, and the three ligands are hydrogen atoms. The prefix “tri-” is used for the hydrogen atoms because there are three of them.
Importance of Correct Spelling
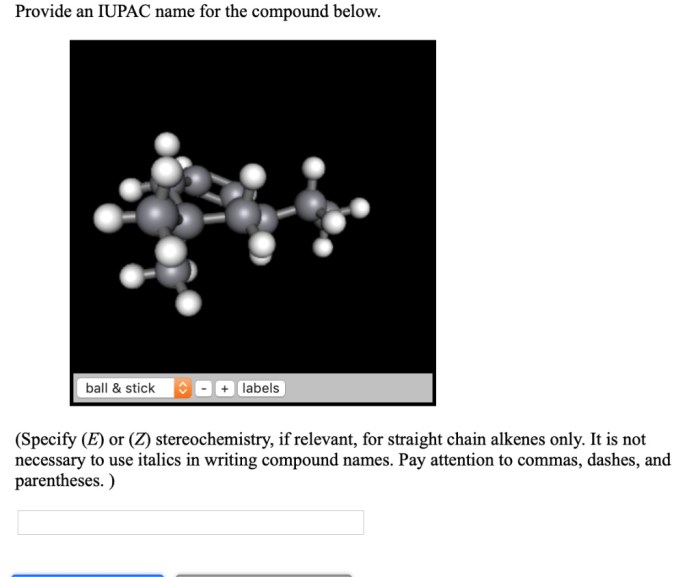
Correct spelling is essential in systematic naming because it ensures that the name of the compound is unambiguous. A single spelling error can change the meaning of the name, which can lead to confusion and errors.
For example, the incorrect spelling “methane” would result in a different compound than the correct spelling “methane”. The incorrect spelling “methane” would refer to a compound with the formula CH3, which is a different compound than methane (CH4).
Incorrect spelling can also lead to errors in communication. If two scientists are using different spellings for the same compound, they may not be able to understand each other, which can lead to confusion and errors.
Common Errors in Systematic Naming
There are a number of common errors that are made in systematic naming. These errors can be avoided by following the IUPAC guidelines for naming covalent compounds.
Some of the most common errors include:
- Using the incorrect prefixes.
- Using the incorrect suffixes.
- Not using the correct order of prefixes and ligands.
- Not using the correct capitalization.
For example, the following are all incorrect names for the compound CH4:
- Methane
- Methan
- Methane
- METHANE
The correct name for the compound CH4 is methane.
Practice Exercises
The following table lists the molecular formula, structural formula, and IUPAC systematic name for a number of covalent compounds.
| Molecular Formula | Structural Formula | IUPAC Systematic Name |
|---|---|---|
| CH4 | H-C-H | H | Methane |
| C2H6 | H-C-C-H | | H H | Ethane |
| NH3 | H-N-H | | Ammonia |
| H2O | H-O-H | Water |
| CO2 | O=C=O | Carbon dioxide |
| NO2 | O=N-O | Nitrogen dioxide |
| SO2 | O=S=O | Sulfur dioxide |
| HCl | H-Cl | Hydrogen chloride |
| HF | H-F | Hydrogen fluoride |
| HI | H-I | Hydrogen iodide |
Students can practice naming covalent compounds by using the table as a reference. Students can also practice naming covalent compounds by using the following website:
IUPAC Nomenclature of Inorganic Compounds
Expert Answers
Why is correct spelling crucial in systematic naming?
Correct spelling ensures the unambiguous identification of compounds, as even minor errors can lead to confusion and misinterpretation.
What are some common errors in systematic naming?
Common errors include incorrect prefixes, missing or misplaced locants, and incorrect use of hyphens and spaces.
How can I avoid errors in systematic naming?
Familiarize yourself with IUPAC guidelines, use reputable resources, and carefully proofread your work.