Embark on a captivating exploration of Post-Lab Module 4: Baking Soda Stoichiometry, a comprehensive guide that delves into the fascinating world of chemical reactions and quantitative analysis. Prepare to unravel the intricacies of stoichiometry, delve into the concept of mole ratios, and master the identification of limiting and excess reactants.
Brace yourself for an immersive learning experience that will transform your understanding of chemical interactions.
Post-Lab Module: Baking Soda Stoichiometry

This post-lab module provides an in-depth exploration of stoichiometry, the study of the quantitative relationships between reactants and products in chemical reactions. Through hands-on experimentation and data analysis, students will gain a comprehensive understanding of stoichiometric principles and their applications.
Learning Objectives
- Understand the concept of stoichiometry and mole ratios.
- Identify limiting and excess reactants in a chemical reaction.
- Calculate the theoretical yield of a reaction based on stoichiometry.
- Compare the actual yield to the theoretical yield and identify sources of error.
Background Information, Post-lab module 4: baking soda stoichiometry
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It involves using mole ratios to determine the exact amounts of reactants and products that are involved in a given reaction.
Mole ratios are ratios of the number of moles of reactants and products in a balanced chemical equation. They provide a way to predict the amount of product that can be obtained from a given amount of reactants.
In a chemical reaction, the reactant that is completely consumed is called the limiting reactant. The reactant that is present in excess is called the excess reactant.
Experimental Procedure
Materials:
- Baking soda (sodium bicarbonate)
- Vinegar (acetic acid)
- Graduated cylinder
- Beaker
- Balance
Steps:
- Measure 100 mL of vinegar into a beaker.
- Weigh out 2.0 g of baking soda.
- Add the baking soda to the vinegar and stir until it dissolves.
- Measure the volume of gas produced using a graduated cylinder.
- Calculate the moles of baking soda used in the reaction.
- Determine the limiting reactant and excess reactant.
- Calculate the theoretical yield of the reaction.
Safety Precautions:
- Wear gloves and safety goggles when handling chemicals.
- Conduct the experiment in a well-ventilated area.
- Dispose of chemicals properly according to laboratory regulations.
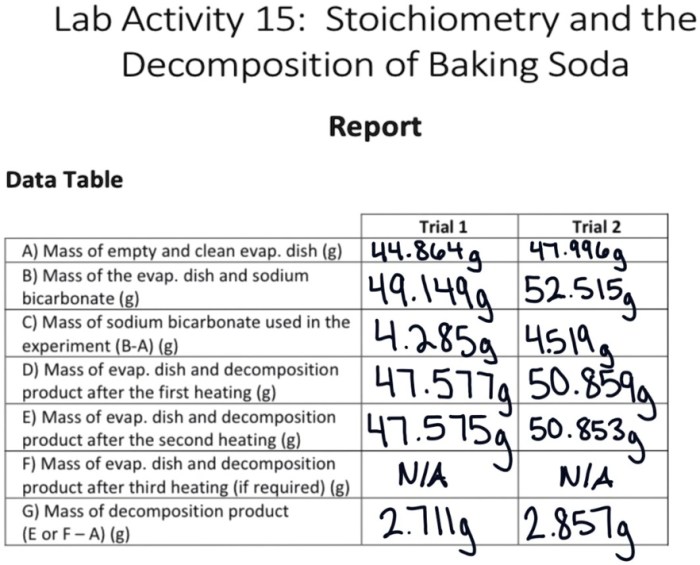
Data Analysis
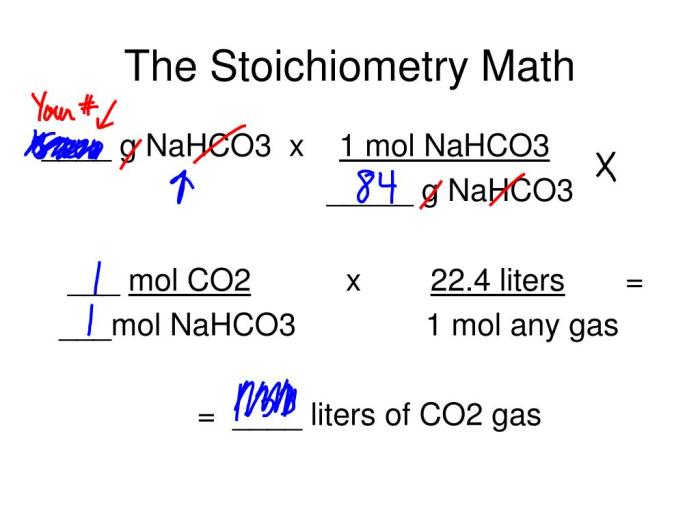
To calculate the moles of baking soda used in the experiment, divide the mass of baking soda by its molar mass (84.01 g/mol).
To determine the limiting reactant, compare the moles of each reactant to their stoichiometric ratio in the balanced chemical equation.
To calculate the theoretical yield of the reaction, multiply the moles of the limiting reactant by the mole ratio of the product to the limiting reactant.
Discussion
Compare the actual yield of the reaction to the theoretical yield. Explain any discrepancies between the actual and theoretical yields.
Discuss the sources of error in the experiment, such as measurement errors, incomplete reactions, and side reactions.
Frequently Asked Questions: Post-lab Module 4: Baking Soda Stoichiometry
What is the purpose of Post-Lab Module 4: Baking Soda Stoichiometry?
This module aims to enhance students’ understanding of stoichiometry, the study of quantitative relationships in chemical reactions, through hands-on experimentation and data analysis.
How does this module help students learn about stoichiometry?
The module provides a comprehensive overview of stoichiometry, including mole ratios, limiting reactants, and excess reactants. Students conduct an experiment to determine the limiting reactant and calculate the theoretical yield of a reaction.
What are the benefits of completing this module?
By completing this module, students will gain a deeper understanding of stoichiometry, develop their experimental skills, and enhance their ability to analyze and interpret data.